Search here
Newspaper
Search here

Arab Canada News
News
By Omayma othmani
Published: January 10, 2024
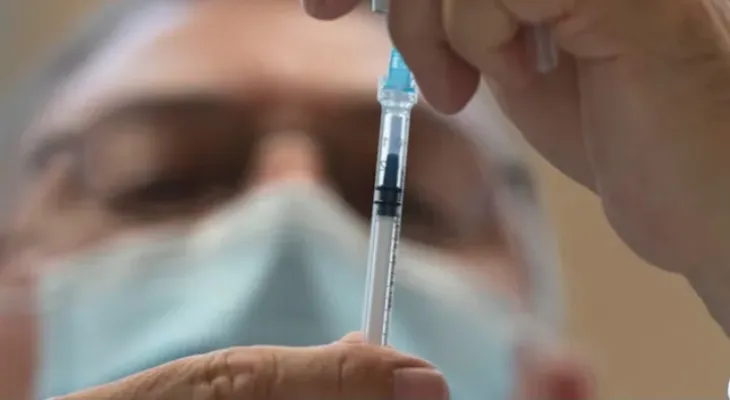
The Canadian Ministry of Health has approved a new vaccine aimed at protecting the two groups most affected by the respiratory syncytial virus (RSV): newborn children, who can receive antibodies through maternal immunization, and Canadians over the age of 60.
This Pfizer Canada bivalent vaccine, called Abrysfo, aims to prevent lower respiratory tract diseases caused by the virus.
This is the first RSV vaccine in Canada approved for use by pregnant women to protect infants from birth up to six months of age, and the second approved for adults aged 60 and over.
Respiratory syncytial virus (RSV) is a common but highly contagious respiratory virus that usually causes cold-like symptoms. For the most vulnerable populations—including infants, the elderly, or those with respiratory or heart diseases—RSV can lead to more severe illness, such as bronchiolitis or pneumonia, and the possibility of hospitalization.
A single dose of the Pfizer vaccine will also be given to pregnant women in the third trimester (from 32 to 36 weeks) who will produce antibodies that can be passed from parent to child. Vaccination during pregnancy is also recommended for other diseases, including COVID-19, influenza, and pertussis.
In August 2023, the Canadian Ministry of Health approved another RSV vaccine for seniors from the manufacturer GSK. In spring 2023, the agency approved an antibody drug called Nirsevimab to protect newborns and infants from severe diseases caused by RSV.
US politics, Canada’s multiculturalism, South America’s geopolitical rise—we bring you the stories that matter.
By signing up, you agree to our Privacy Policy
The police force in Canada's largest province by population and economic size announced that its Fre...
19 March 2024
Canadian Foreign Minister Mélanie Joly expressed her concern about the "fraudulent presidential elec...
19 March 2024
The Toronto Public Health Agency said that an infant who recently returned from travel contra...
19 March 2024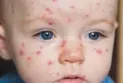
The office of Canadian Prime Minister Justin Trudeau said that the Prime Minister expressed his conc...
19 March 2024
A Canadian-born commander of the so-called Norman Brigade – a volunteer combat group in Ukraine – ha...
19 March 2024
Vaughan Mayor Steven Del Duca is calling on the city to approve a bylaw banning protests outside pla...
19 March 2024


Thursday, 03 July 2025
--°C
--°C
Comments