Search here
Newspaper
Search here

Arab Canada News
News
Published: October 13, 2022
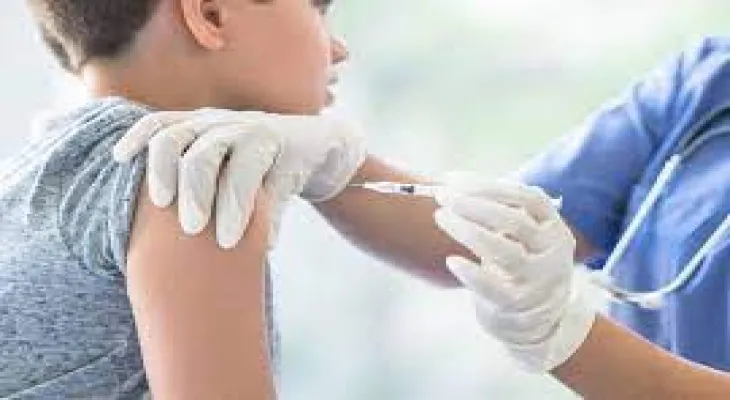
Residents of Ontario aged 12 and over will soon be able to receive a bivalent COVID-19 booster as shipments of the newly approved Pfizer vaccination series are scheduled to arrive in the province this week.
The Canadian Ministry of Health approved Pfizer’s bivalent vaccine on Friday, targeting the BA.4 and BA.5 lineages of the Omicron variant.
Previously, the Moderna bivalent booster – available to those aged 18 and over – was approved and available in Ontario.
The Canadian Ministry of Health stated that the bivalent booster drug has already been given to nearly five million people in the United States with no new safety concerns.
The Ministry of Health recommends most Ontario residents under 65 receive a booster dose six months after their last vaccination.
For high-risk individuals, the recommended interval between vaccinations is shortened to three months.
Dr. Kieran Moore, Ontario’s Chief Medical Officer of Health, said in a government statement issued Thursday: “We are entering the fall season where we traditionally see an increase in respiratory illness cases, including COVID-19.”
“Vaccines improve your immune response and reduce the risk of severe illness, hospitalization, and post-infection symptoms.
We recommend Ontario residents stay up to date on their COVID-19 vaccines at the appropriate interval since their last dose, and get the annual flu vaccine when it becomes available.”
Appointments for a dose of Pfizer’s bivalent vaccine can be booked via the province’s vaccination booking portal, public health units, the regional vaccine call center at 1-833-943-3900, or participating pharmacies.
The earliest appointment can be scheduled for October 17. The province also announced today that Ontario residents can receive flu vaccines from local healthcare providers starting November 1.
The approval of Pfizer’s bivalent boosters comes after the Canadian Ministry of Health approved the manufacturer’s new children’s vaccine for those aged six months to five years, providing an alternative to the Moderna shot used since July.
Comments